Base editing enables precise modifications to DNA by converting one DNA base pair to another, without introducing double-strand breaks. This innovative technology offers unparalleled accuracy in genome editing, opening new possibilities for targeted genetic modifications with reduced off-target effects.
Base editors are molecular tools capable of precisely altering the genetic code by changing individual bases within the DNA sequence.
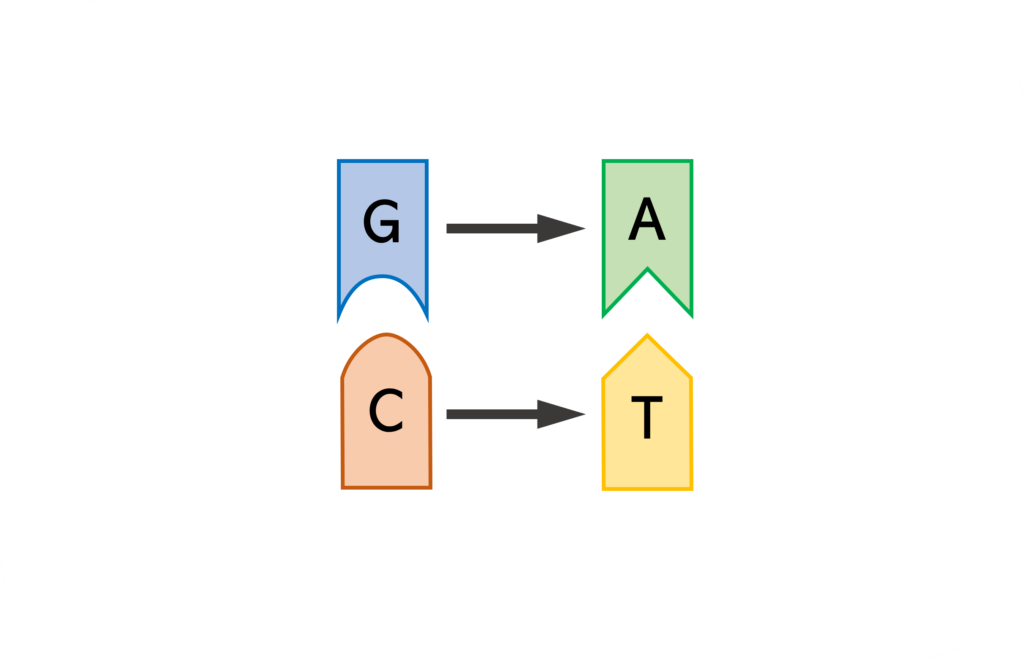
TAM base editor contains a fusion protein of nickase Cas9 and activation-induced cytidine deaminase (AID) with/without UGI , and a single guide RNA (sgRNA). TAM base editing tool can convert C:G base pair to T:A base pair at DNA level.
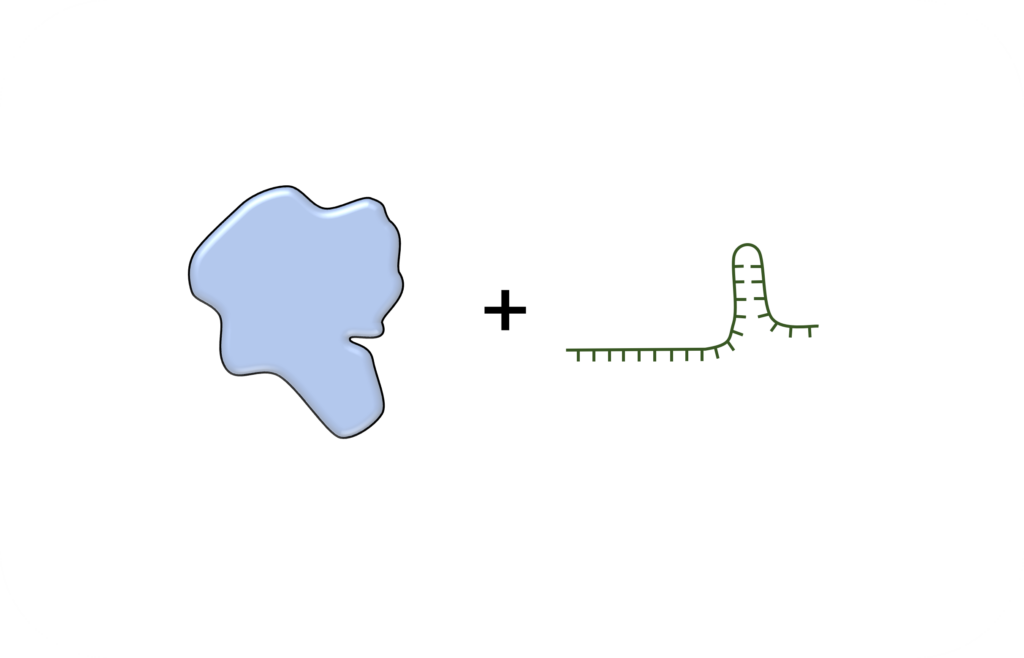
DNA mutations underlie over 8000 known genetic diseases. Base editing holds promise as a potential effective treatment.
Senior pharmacology and toxicology advisor

Pharmacology, Shang University of Traditional Chinese Medicine.
Senior Vice General Manager at Shanghai InnoStar Biotechnology Co., Ltd.
More than 20 years in preclinical pharmacology and toxicology evaluation of new drugs.
Senior Scientific Advisor

Ph.D., Molecular Cell Biology and Bioengineering, Max Planck Research School.
Senior Director of genome engineering department, AstraZeneca, Gothenburg, Sweden.
More than 10 years’ experience in gene editing tool and drug development.
Senior Clinical Physician Advisor

Ph.D. , Peking Union Medical College, Tsinghua University.
Associate Chief physician. Neurology Department. Peking Union Medical College Hospital, Beijing.
Member of the Neurology Branch of the Chinese Medical Association.
Member of the DMD/BMD Study Group of the Chinese Rare Disease Alliance.
Specializes in complex and rare neurological disorders, gene diagnosis of monogenic.
Senior strategic Advisor

Over 30 years of experience in drug discovery, clinical translation, clinical practice, and operational management Former President of Chongqing Medical and Pharmaceutical College.
Ex-Vice President of the Technical Center at Fosun Pharma.
Senior Biostatistics Advisor

Ph.D. Biostatistics, University of Michigan, USA.
Bettyann Asche Murray Distinguished Professor, Deputy Chair.
Department of Biostatistics, University of Texas, MD Anderson Cancer Center.
Inventor of Bayesian Optimal lnterval PhaseⅠ/ⅡTrial Design (BOlN12); Participated in clinical trial design of many anti-cancer treatment.
Co-funder, Senior scientific Advisor

Ph.D. , Pathology, Ohio State University
Associate Professor at West Lake University, Young Scientist in the 973 Program
Thousand Youth Talents Program, National Outstanding Young Scientist
Inventor of Targeted AID-mediated Mutagenesis (TAM) base editor
CEO, Co-Founder

Ph.D. , Immunology, Shanghai Jiao Tong University.
MBA, Manchester Business School.
Over 20 years of experience in pharmaceutical industry, covering R&D, BD, Investment and Incubation.
Quality management advisor

Former Director of the Quality Assurance Department and Laboratory at the Shanghai Institute of Biological Products
Research and establishment of quality standards for various viral bioproducts as well as registration applications
Committee Member of the Chinese Pharmacopoeia Commission,
Expert Member of the Shanghai Science and Technology Commission
Committee Member of the Quality Management Committee of the Shanghai Medical Industry Association
Member of the New Technology/Skills Professional Committee.